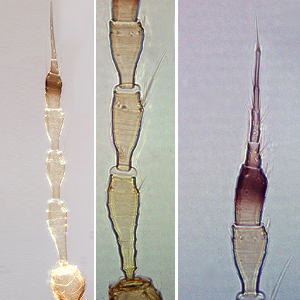
Heliothrips haemorrhoidalis (Bouche, 1833)
Panchaetothripinae, Thripidae, Terebrantia, Thysanoptera
Figures
Fig. 1: 8-segmented antenna, segments III & IV with simpe sense cone, terminal segments V-VIII
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Pronotum
Fig. 4: Meso- and metanotum
Fig. 5: Metanotum and tergites I & II
Fig. 6: Fore wing and fore wing basal region
Fig. 7: Tergites III-V
Fig. 8: Tergites VIII-XI
Fig. 9: Tergites VIII-IX, microtrichia comb
Fig. 10: Ovipositor, tergites VIII-XI
Fig. 11: Color variations of adult females
Introduction and recognition
Heliothrips haemorrhoidalis is a polyphagous, leaf feeding thrips and causes damage on plants with rather hard leaves, including tea, coffee, citrus and avocado. Both sexes fully winged. Body dark brown when mature, but abdomen golden in less mature adults (Fig. 11); legs yellow; antennal segments III-V and VII & VIII yellow, VI brown in apical half, I & II light brown; fore wings pale with hind margin shaded. Antennae 8-segmented; segments III & IV with sense cone simple, VIII at least 3 times as long as VII (Fig. 1). Head wider than long, strongly reticulate; cheeks slightly concave but constricted at base (Fig. 2). Pronotum reticulate; with no long setae (Fig. 3). Mesonotum with an incomplete median division. Metanotum with strongly reticulate triangle; median setae small on anterior half of sclerite (Fig. 4 and 5). Mid and hind tarsi short and 1-segmented. Fore wing slender with apex rounded bearing 2 long cilia; fore wing first vein close to or fused to costal vein, costa with long cilia, posteromarginal cilia not wavy; veinal setae not much larger than surface microtrichia (Fig. 6). Tergites covered by large hexagonal reticulations except for a pair of posterior submedian smooth areas; tergites II-VIII with 1 pair of dominant setae medially (Fig. 7); VIII with posteromarginal comb of long and slender microtrichia (Fig. 8 and 9); X short but with complete median division (Fig. 10). Sternites with 3 pairs of small marginal setae.
Male very rare; similar to female but smaller in size; tergite IX with 3 pairs of stout thorn-like setae; sternites III-VII each with a transverse oblong glandular area.
Taxonomic identity
Species
Heliothrips haemorrhoidalis (Bouche, 1833)
Taxonomic history
Dinurothrips rufiventris Girault, 1929
Heliothrips semiaureus Girault, 1928
Heliothrips angustior Priesner, 1923
Heliothrips ceylonicus Schmutz, 1913
Heliothrips abdominalis Reuter, 1891
Thrips adonidumCook, 1873
Heliothrips adonidum Haliday, 1836
Thrips haemorrhoidalis Bouche, 1833
Common name
Greenhouse thrips
Black greenhouse thrips
Black tea thrips
Present taxonomic position
Family: Thripidae Stephens, 1829
Subfamily: Panchaetothripinae Bagnall, 1912
Genus: Heliothrips Haliday, 1836
Genus description
The genus Heliothrips Haliday, 1836
There are 4 species within this genus, one of each from South Africa, Indonesia and South America, and the greenhouse thrips, Heliothrips haemorrhoidalis, that is now distributed worldwide (Mound & Kibby 1998). All of the species are flattened dorso-ventrally and show heavy reticulation. The head is covered with polygonal reticulations and has a constricted neck, antennae are 8-segmented, antennal segments III & IV have a simple sense cone, fore wings have straight cilia on the posterior margin, minute setae on veins, and a rounded apex and a swollen base (Mound & Marullo 1996; Wilson 1975).
Species description
Typical key character states of Heliothrips haemorrhoidalis
Coloration and body sculpture
Surface of head, pronotum and fore legs: with heavy, often polygonally reticulate sculpture
Sculptured reticles on head and pronotum: with no internal markings
Body color: mainly brown to dark brown, rare misinterpreted as bicolored
Antennae
Number of antennal segments: 8
Form of sense cones on antennal segment III and IV: emergent and simple on segment III and IV
Terminal antennal segments: VI-VIII forming a single unit
Head
Cheeks shape: constricted to basal neck
Head - occipital ridge dorsally: absent
Ocelli: present
Head: not prolonged in front of compound eyes (misinterpreted: distinctly prolonged)
Head length to wide: length < width
Prothorax
Pronotal blotch or internal apodeme: absent
Pronotum shape: broadly rectangular
Pronotum surface: with mainly equiangular reticulations
Mesothorax
Mesonotum: with an incomplete median division
Metathorax
Metanotum with dominant sculptured triangle medially: with dominant sculptured triangle medially
Shape of metathoracic furca: transverse, V-shaped
Wings
Fore and hind wings: present, more than half as long as abdomen (macropterous)
Fore- and hind wing surface: covered with microtrichia
Fringe cilia arising: from sockets
Fore wing veins: present
Apex of fore wing: with prominent terminal setae
Fore wing anterior margin (costal vein): with cilia but minute setae or without setae
Fore wing costal fringe cilia: arising at anterior margin of wing
Fore wing first vein: close to or fused to costal vein
Fore wing first vein setal row: incomplete, with setae not closely and uniformly spaced
Fore wing second vein setal row: incomplete, with setae not closely and uniformly spaced
Fore wing shape: mainly parallel sided or margins run continuously towards each other
Fore wing surface: not reticulate
Fringe cilia on posterior margin near apex: straight
Length of fore wing costal setae at middle of wing: minute
Fore wing: pale with dark area(s), or pale, with posterior margin shaded or uniformly pale or weakly shaded
Fore wing extreme apex color: pale
Shape of fore wing apex: with mainly posterior margin curved to join anterior margin
Legs
Color of fore tarsi: pale or yellow, sometimes apical shaded or brown
Mid and hind tarsi: with one segment
Abdomen
Tergite II: without numerous recurved claw-like microtrichia anterolaterally
Tergites IV and V median setal pair: longer than distance between their bases
Tergites V to VII: without ctenidia laterally, but sometimes with rows of microtrichia
Tergite VIII to X: without unusually long and stout setae
Tergites: without distinctive tergal sculpture forming a series of arches on the antecostal ridges
Tergite X: not tubular, longitudinally incomplete
Setae on abdominal tergite X: all setae slender

Similar or related species
The species is similar to Heliothrips sylvanus that differs from Heliothrips haemorrhoidalis in lacking fringe cilia on anterior margin of fore wings, having parallel cheeks on head and entirely dark brown legs. The head is more or less as long as wide, the median setal pair of tergites is shorter than distance between their bases, and tergite VIII has a posteromarginal comb of short microtrichia. Heliothrips haemorrhoidalis with distinct fringe cilia on anterior margin of fore wings, cheeks slightly concave, head wider than long, legs pale, the median setal pair of tergites is longer than distance between their bases, and tergite VIII with a posteromarginal comb of laterally long and slender microtrichia, the long median microtrichia originate on the anterior margin of tergite IX.
Heliothrips is one of several small genera of Panchaetothripinae in which the species have slender fore wings with very short major setae and straight posteromarginal cilia without undulations, like in Phibalothrips, Parthenothrips, Retithrips and Rhipiphorothrips. Compared to Phibalothrips peringueyi, the species of Heliothrips have 8-segmented antennae and often a complete comb of posteromarginal microtrichia on tergite VIII (Phibalothrips peringueyi with 7-segmented antennae, and only laterally microtrichia on tergite VIII). Retithrips syriacus differs from others in having a reticulate sculpture that bears internal markings, the shape of metathoracic furca is elongate and Y-shaped, fore wings bearing anteromarginally 3 curious blister-like callosities, and minute setae on tergite X. Rhipiphorothrips miemsae differs from species of Heliothrips and other species in bearing a strong irregular, reticulate and rugose sculpture on head and pronotum, and having a complete longitudinal division of the mesonotum. Furthermore, Retithrips as well as Rhipiphorothrips have a forked sense cone on antennal segments III & IV (Heliothrips and Phibalothrips with simple sense cone on antennal segments III & IV).
Biology
Life history
As with other thrips species the life cycle from egg to adult is dependent on temperature. The duration of its life cycle has been reported to be 30-32 days at 26-28°C, 40 days at 23-25°C (Ananthakrishnan 1971); total life-cycle takes from 8 weeks at 19°C to 12 weeks at 15°C (Hill 1983). More than 12 generations per year have been reported.
Host plants
Polyphagous.
Crops: avocado, banana, citrus, cocoa, coconut, coffee, cotton, date palm, granate apple, grape vine, mango, passion fruit, peach, tea.
Vector capacity
Can transmit Puccinia graminus uredia on cereal.
Damage and symptoms
Primarily feeds on the underside of leaves. Leaves become distorted, curled under, silvered and covered by black spots on the undersides, and turn brown (Hill 1983). Plants become stunted, flowers become discolored. Greenhouse thrips commonly infests the fruits in areas of contact between touching avocado fruits, rather than on single fruits. Fruit surfaces become bronzed and under severe infestation, fruit cracking occurs. Damaged fruits are unsuitable for export. In South Africa, combined infestation of greenhouse thrips and red banded thrips could result in losses of up to 80% (Dennill & Erasmus, 1991).
Detection and control strategies
Monitoring of greenhouse thrips on fruits such as avocado in the early season infestation should be focussed on fruits that are touching each other than on single fruits (Dennill and Erasmus, 1992a). Cultivar "Hass" are more susceptible to thrips infestation (Erichsen and Schoeman, 1992). Only one effective natural enemy is known to attack greenhouse thrips, the minute larval parasite Thripobius javae (=Thripobius semiluteus), which was introduced into California from Brazil and Australia in the mid-1980s. Parasitized thrips larvae appear swollen and the sides of their body are more parallel than tapered as in the case of healthy thrips larvae. The immobile parasite pupae appear black among the colonies of translucent, unparasitized thrips (Bernardo et al. 2005). Other less effective natural enemies include an egg parasite, Megaphragma mymaripenne, and three predatory thrips species, Franklinothrips orizabensis, Franklinothrips vespiformis, and Leptothrips mali, also known as the black hunter (Denmark & Fasulo 1967).
Additional notes
As the common name implies, the greenhouse thrips is a pest in greenhouses in temperate countries. Under field conditions it is usually found on plants with rather hard leaves (not on herbs or soft leaves), including tea, Pinus and avocado in parts of Africa (Mound & Monteiro 1998; Scott Brown & Simmonds 2006). Adults, larvae and pupae are sometimes abundant on older senescing leaves, often on plants growing suboptimally. Heliothrips haemorrhoidalis is a slow moving insect. The larvae are small and pale in color compared to the adults. Adults and larvae can be found carrying a small black fecal drop on the tip of their abdomen. This drop is thrown in defense against predators.
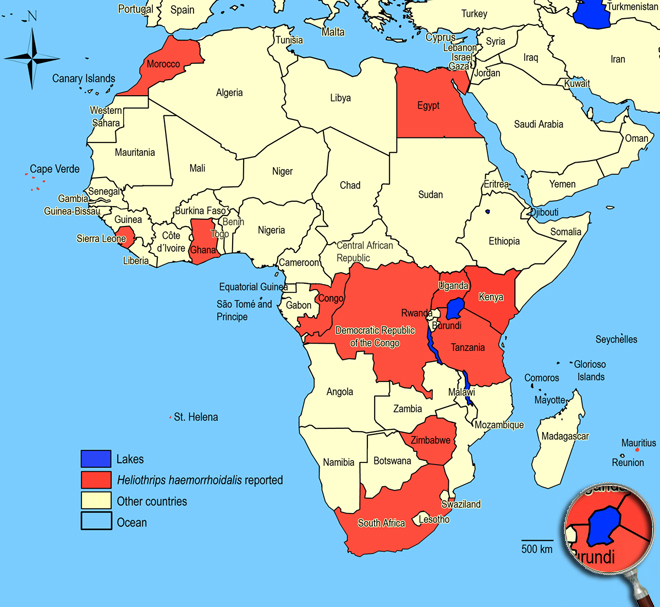
Biogeography
Originally from southwestern Brazil, the greenhouse thrips is now found throughout the tropics and subtropics, in warmer regions and greenhouses (cosmopolitan). Africa, Asia, Australia, New Zealand, Central and South America, Europe, North America. Cape Verde Islands (Santo Antão - Ribeira de Paul),
Egypt (Alexandria),
Ghana,
Kenya (Thika, Mbita and Taita),
Sierra Leone,
South Africa,
St. Helena,
Uganda.
African countries where Heliothrips haemorrhoidalis has been reported
Occurence of Heliothrips haemorrhoidalis in East Africa
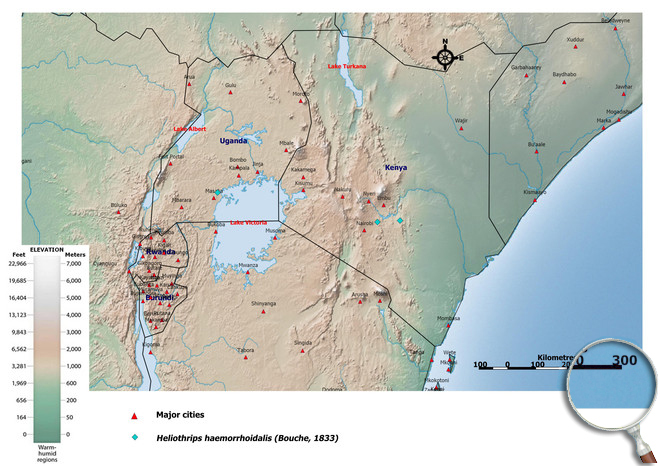
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Click here for locations of Heliothrips haemorrhoidalis in parts of East Africa.

Bibliography
Ananthakrishnan TN (1971). Thrips (Thysanoptera) in agriculture, horticulture & forestry - diagnosis, bionomics & control. Journal of Scientific and Industrial Research. 30 (3): 113-146
Bernardo U, Viggiani G & Sasso R (2005). Biological parameters of Thripobius semiluteus Boucek (Hym., Eulophidae), a larval endoparasitoid of Heliothrips haemorrhoidalis (Bouche) (Thysan., Thripidae). Journal of Applied Entomology. 129 (5): 250-257
Bouché PF (1833). Naturgeschichte der schädlichen und nützlichen Garten-Insekten und die bewährtesten Mittel zur Vertilgung der ersteren. Nicolaische Buchhandlung, Berlin, 178 pp
Burmeister H (1836). Handbuch der Entomologie, Verlag Enslin, Berlin, Vol. 2 (2), pp. 397-1050
Childers CC & Nakahara S (2006). Thysanoptera (thrips) within citrus orchards in Florida: Species distribution, relative and seasonal abundance within trees, and species on vines and ground cover plants. Journal of Insect Science. 6 (45): 1-19
Crawford JC (1940). The male of Heliothrips haemorrhoidalis (Bouché) (Thysanoptera). Proceedings of the Entomological Society of Washington. 42 (4): 90-91
Daniel AM & Chandrasekar SS (1986). Insect-fern interactions with particular reference to Heliothrips haemorrhoidalis (Bouche) (Thysanoptera, Panchaetothripinae). Current Science. 55 (14): 676-678
del Bene G, Cavallo V, Lupetti P & Dallai R (1998). Ultrastructure of the accessory gland in the parthenogenetic thrips Heliothrips haemorrhoidalis (Bouche) (Thysanoptera: Thripidae). International Journal of Insect Morphology & Embryology. 27 (3): 255-261
del Bene G, Cavallo V, Lupetti P & Dallai R (1999). Fine structure of the salivary glands of Heliothrips haemorrhoidalis (Bouche) (Thysanoptera: Thripidae). International Journal of Insect Morphology & Embryology. 28 (4): 301-308
Denmark HA & Fasulo TR (1967). Greenhouse thrips, Heliothrips haemorrhoidalis (Bouche). Entomology Circular No. 64. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, 3 pp http://edis.ifas.ufl.edu/in232
Dennill GB (1992). Orius thripoborus (Anthocoridae), a potential biocontrol agent of Heliothrips haemorrhoidalis and Selenothrips rubrocinctus (Thripidae) on avocado fruit in the eastern Transvaal. South African Avocado Growers' Association Yearbook 1992. 15: 55-56
http://www.avocadosource.com/Journals/SAAGA/SAAGA_1992/SAAGA_1992_PG_55-56.pdf
Dennill GB & Erasmus MJ (1991). A packhouse survey of insect damage to avocado in the Nelspruit/Hazyview area during 1990. South African Avocado Growers Association Yearbook 14, 79 - 82.
Dennill GB & Erasmus MJ (1992a). Basis for a practical technique for monitoring thrips in avocado orchards. Crop Protection. 11 (1): 89-91
Dennill GB & Erasmus MJ (1992b). The insect pests of avocado fruits - Increasing pest complex and changing pest status. Journal of the Entomological Society of Southern Africa. 55 (1): 51-57
Erichsen C & Schoeman A (1992). Economic losses due to insect pests on avocado fruit in the Nelspruit/Hazyview region of South Africa during 1991. South African Avocado Growers’ Association Yearbook 1992. 15: 49-54
Girault AA (1929). New pests from Australia VI. Published privately, Brisbane, 4 pp
Haliday AH (1836). An epitome of the British genera, in the order Thysanoptera, with indications of a few of the species. The Entomological Magazine. 3 (5): 439-451
Hessein NA & McMurtry JA (1988). Observations on Megaphragma mymaripenne Timberlake (Hymenoptera, Trichogrammatidae), an egg parasite of Heliothrips haemorrhoidalis Bouche (Thysanoptera, Thripidae). Pan-Pacific Entomologist. 64 (3): 250-254
Hessein NA & McMurtry JA (1989). Biological studies of Goetheana parvipennis (Gahan) (Hymenoptera, Eulophidae), an imported parasitoid, in relation to the host species Heliothrips haemorrhoidalis (Bouche) (Thysanoptera, Thripidae). Pan-Pacific Entomologist. 65 (1): 25-33
Hill D (1983). Agricultural insect pests of the tropics and their control, (2nd edition). Cambridge University Press, Cambridge, 746 pp
Hoddle MS (2003). Predation behaviors of Franklinothrips orizabensis (Thysanoptera: Aeolothripidae) towards Scirtothrips perseae and Heliothrips haemorrhoidalis (Thysanoptera: Thripidae). Biological Control. 27 (3): 323-328
Kudo I (1992). Panchaetothripinae in Japan (Thysanoptera, Thripidae), 1. Panchaetothripini, the genera other than Helionothrips. Japanese Journal of Entomology. 60 (1): 109-125
Lewis T (1973). Thrips: their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp
Lewis T (1997). Thrips as crop pests. CAB International, Wallingford, 740 pp
Miyazaki M & Kudo I (1989). Descriptions of thrips larvae which are noteworthy on cultivated plants (Thysanoptera). II. Pseudodendrothrips mori (NIWA). Applied Entomology and Zoology. 24 (2): 209-212
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Mound LA (1976). The identity of the greenhouse thrips Heliothrips haemorrhoidalis (Bouché) (Thysanoptera) and the taxonomic significance of spanandric males. Bulletin of Entomological Research. 66 (1): 179-180
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp
Mound LA & Marullo R (1996). The thrips of Central and South America: An introduction (Insecta: Thysanoptera). Memoirs on Entomology, International, Vol. 6. Associated Publishers, Gainsville, 487 pp
Mound LA, Marullo R & Trueman JWH (2001). The greenhouse thrips, Heliothrips haemorrhoidalis, and its generic relationships within the subfamily Panchaetothripinae (Thysanoptera: Thiripidae). Insect Systematics & Evolution. 32 (2): 205-216
Mound LA & Monteiro RC (1997). A review of the genus Heliothrips (Thysanoptera; Thripidae), with a new sister-species of the greenhouse thrips from south eastern Brazil. Journal of the New York Entomological Society. 105 (3-4): 154-160
Palmer JM (1990). Identification of the common thrips of Tropical Africa (Thysanoptera, Insecta). Tropical Pest Management. 36 (1): 27-49
Palmer JM, Mound LA & du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Pintureau B, Lassabliere F, Khatchadourian C & Daumal J (1999). Eggs parasitoids and symbionts of two European thrips. Annales de la Société Entomologique de France. 35 (supplément): 416-420
Pitkin BR & Mound LA (1973). A catalogue of West African Thysanoptera. Bulletin de ľInstitut Fondamental ďAfrique Noire, Série A. 35 (2): 407-449
Priesner H (1923). Ein Beitrag zur Kenntnis der Thysanopteren Surinams. Tijdschrift voor Entomologie. 66: 88-111
Priesner H (1926-28). Die Thysanopteren Europas. F. Wagner Verlag, Wien, 755 pp
Priesner H (1938). Contributions towards a knowledge of the Thysanoptera of Egypt, XI. Bulletin de la Société Royale Entomologique ďEgypte. 21: 208-222
Rivnay E (1935). Ecological studies of the greenhouse thrips, Heliothrips haemorrhoidalis, in Palestine. Bulletin of Entomological Research. 26 (2): 267-278
Russell HM (1909). The greenhouse thrips. Bulletin, USDA, Bureau of Entomology. 64 (6): 43-60
Schmutterer H (1998). Some arthropod pests and a semi-parasitic plant attacking neem (Azadirachta indica) in Kenya. Anzeiger für Schädlingskunde, Pflanzenschutz, Umweltschutz. 71 (2): 36-38
Scott Brown AS & Simmonds MSJ (2006). Leaf morphology of hosts and nonhosts of the thrips Heliothirps haemorrhoidalis (Bouché). Botanical Journal of the Linnean Society. 152 (1): 109-130
Scott Brown AS, Simmonds MSJ & Blaney WM (1999). Influence of species of host plants on the predation of thrips by Neoseiulus cucumeris, Iphiseius degenerans and Orius laevigatus. Entomologia Experimentalis et Applicata. 92 (3): 283-288
Scott Brown AS, Simmonds MSJ & Blaney WM (2002). Relationship between nutritional composition of plant species and infestation levels of thrips. Journal of Chemical Ecology. 28 (12): 2399-2409
Scott Brown AS, Simmonds MSJ & Blaney WM (2003). Influence of a short exposure to teflubenzuron residues on the predation of thrips by Iphiseius degenerans (Acari: Phytoseiidae) and Orius laevigatus (Hemiptera: Anthocoridae). Pest Management Science. 59 (11): 1255-1259
Wilson TH (1975). A monograph of the subfamily Panchaetothripinae (Thysanoptera: Thripidae). Memoirs of the American Entomological Institute. 23: 1-354
zur Strassen R (1960). Catalogue of the known species of South African Thysanoptera. Journal of the Entomological Society of Southern Africa. 23 (2): 321-367
zur Strassen R (1969). Neue Angaben zur Thysanopteren-Fauna (Insecta, Thysanoptera) der Kanarischen Inseln. Commentationes Biologicae. 31 (5): 1-74
zur Strassen R (1976). La fauna terrestre de ľile de Sainte-Hélène III - 18. Thysanoptera. Annales de la Musee Royal de ľAfrique Central, Série 8, Sciences Zoologiques. 215: 236-256
zur Strassen R (1980). Thysanopterologische Notizen (5) (Insecta: Thysanoptera). Senckenbergiana Biologica. 60 (3-4): 191-202
zur Strassen R (2003). Die terebranten Thysanopteren Europas und des Mittelmeer-Gebietes. Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise, 74. Teil. Goecke & Evers, Keltern, Germany, 277 pp.
zur Strassen R (2006). Checklist of the Thysanoptera (Insecta) of southern Africa. African Entomology. 14 (1): 63-68
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California